TK and JPB Co-first; RK and AMZ are Co-senior authors.
Introduction
Recognition of the growing importance of genetic mutations in prognostication for pts with MDS prompted the development of IPSS-M to improve outcome prediction. Subsequent validation studies included a heterogenous groups of pts; treated & untreated pts with lower & higher risk (HR) disease, and thus the value of IPSS-M in predicting outcomes of pts with HR-MDS treated with HMA remains unclear. We aimed to evaluate the ability of IPSS-M to predict outcomes of pts with HR-MDS who were treated with HMA in a large multicenter cohort.
Methods
Using the multi-center VALIDATE database, we included pts with HR-MDS (Defined as IPSS score >1 or IPSS-R score > 3.5) who were treated with any HMA in the frontline setting and had available molecular data before HMA initiation. Time-to-event analysis used Kaplan-Meier estimator and the log-rank tests. Survival was measured from time of HMA initiation. We compared the different scoring systems by Harrell's c-index. Best response to HMA was assessed using the IWG 2023 response criteria in HR pts (n=480) who had a marrow evaluation within 180 days of HMA initiation. This study was supported by an independent research grant from AbbVie.
Results
A total of 925 pts were included in the analysis. Median age was 68 years (IQR: 62-75) and 66% of the pts were men. MDS excess blast 1/2 accounted for 65% of the diagnosis. Overall, 76% of pts were treated with HMA monotherapy (29% decitabine and 71% azacitidine), 24% received HMA combination therapy, and 39% of pts had HSCT. The most common mutations (MT) were TP53 (32%), ASXL1 (20%), TET2 (13%) and SRSF2 (12%); 36% of pts had MDS harboring >1 gene MT.
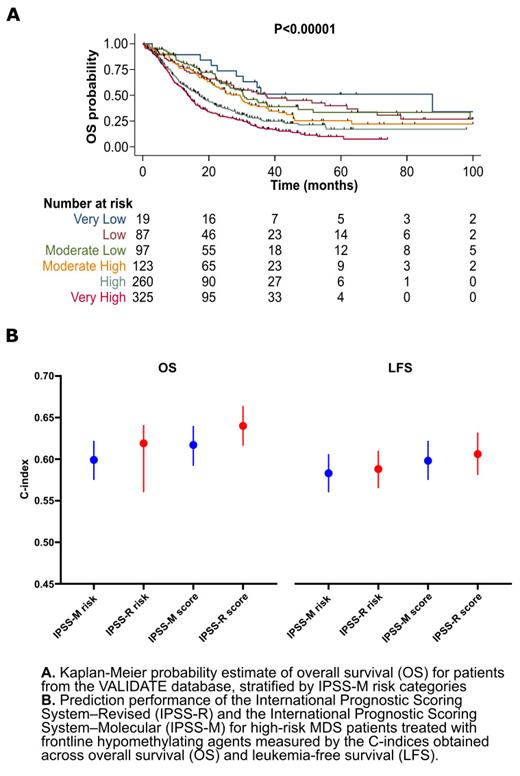
As anticipated, the cohort was enriched with high risk (26%) and very high risk (46%) IPSS-R groups. According to pre-therapy IPSS-M, pts were classified into very low (2%), low (9%), moderate low (11%), moderate high (14%), high (29%) and very high (36%) risk groups. IPSS-M reclassified 54% of pts, of whom 184 (37%) were up-staged and 317 (63%) were down-staged. Specifically, 38%/21% of pts with intermediate risk IPSS-R MDS, and 30%/36% of pts with high risk IPSS-R MDS were up-staged/down-staged, respectively. In addition, 44% of pts with very high risk IPSS-R MDS were down-staged.
The median follow-up time was 19 (IQR:10-35) months (mo). Median overall survival (OS) was 19 (95% CI: 17-22) mo. Overall, 35% of the pts progressed to AML during follow up. IPSS-M groups showed significant differences for both OS and LFS (p-value: <0.0001 for both). The median OS (mo, 95%CI) based on IPSS-M categories were: low (37, 25-62), moderate low (30, 23-47), moderate high (29, 22-35), high (17, 14-20), and very high (14, 12-15) (Panel A). The 3-year LFS (%) were: low (20%), moderate low (48%), moderate high (41), high (50%), and very high (59%). IPSS-M was significantly associated with OS in a multivariable model adjusted for age, sex, type of HMA, number of HMA cycles and receiving allogeneic HSCT (HR: 1.4, 95% CI:1.2-1.5).
However, IPSS-M showed comparable performance when compared with the IPSS-R with c-index (95%CI): 0.599 (0.557-0.622) vs. 0.619 (0.560-0.641) for OS. In addition, IPSS-M showed similar performance for LFS: 0.583 (0.560-0.606) vs. 0.588 (0.565-0.610) (Panel B). When IPSS-M/IPSS-R were used as continuous scores, the c-indices (95%CI) for OS and LFS were: 0.617 (0.592-0.640) vs. 0.6400 (0.616-0.664) for OS and 0.598 (0.575-0.622) vs. 0.606 (0.581-0.632) for LFS. We then analyzed the performance of IPSS-R/IPSS-M in pts with HR MDS (IPSS ≥1.5). Both IPSS-R and IPSS-M had comparable performance for OS prediction (c-index:0.548 vs. 0.566) and LFS (0.551 vs. 0.538).
Based on the proposed IWG 2023 response criteria, complete remission rate (CR)/ overall response rate (ORR) were 12/47%, 9/58%, 13/47%, and 17/57% of pts assigned to moderate low, moderate high, high, and very high IPSS-M risk groups. No significant association was observed between IPSS-M and ORR (OR: 1.1, p=0.475) or CR (1.1, p=0.286).
Conclusions
In our multi-center large cohort of pts with HR MDS treated with frontline HMA, IPSS-M significantly stratified pts for OS and LFS. However, when compared with IPSS-R, IPSS-M did not seem to substantially improve prediction of outcomes among HMA-treated pts. Our results emphasize the high predictive prognostic power of clinical features and cytogenetic alterations among HR-MDS pts treated with HMA.
Disclosures
Stahl:Kymera: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Rigel: Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: GME activity ; Dedham group: Consultancy; Curis Oncology: Other: GME activity ; Haymarket Media: Other: GME activity ; Clinical care options: Other: GME activity ; Boston Consulting: Consultancy. DeZern:Geron: Membership on an entity's Board of Directors or advisory committees; Caribou: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy; Appellis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Sobi: Consultancy. Sekeres:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Geron: Membership on an entity's Board of Directors or advisory committees; Kurome: Consultancy, Current holder of stock options in a privately-held company; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees. Carraway:Agios: Consultancy, Speakers Bureau; Astex Pharmaceuticals: Other; Syndax: Other: DSMB; Celgene: Research Funding; Jazz Pharmaceuticals: Consultancy, Other: Travel, Accommodations, Expenses , Speakers Bureau; Takeda: Other; BMS: Consultancy, Research Funding, Speakers Bureau; Stemline Therapeutics: Consultancy, Speakers Bureau; AbbVie: Other; Genentech: Consultancy; Daiichi: Consultancy; Novartis: Consultancy, Other: Travel, Accommodations, Expenses , Speakers Bureau. Desai:BMS: Consultancy, Other: Advisory role; Janssen Research & Development: Research Funding; Abbvie: Consultancy, Other: Advisory role; Janssen Pharmaceuticals: Current Employment; Servier: Consultancy, Other: Advisory role. Griffiths:Novartis: Consultancy, Research Funding; Artis Ventures: Membership on an entity's Board of Directors or advisory committees; MDS International Foundation: Honoraria; Celldex Therapeutics: Research Funding; AAMDSIF: Honoraria; CTI Biopharma: Consultancy; AstraZeneca Rare Disease: Consultancy, Research Funding; Takeda Oncology: Consultancy; NextCure, Inc: Research Funding; Vera and Joseph Dresner Foundation: Membership on an entity's Board of Directors or advisory committees; S. Karger Publishing: Honoraria; Alexion Pharmaceuticals: Consultancy, Research Funding; Abbvie: Consultancy; Taiho Oncology: Consultancy; Apellis Pharmaceuticals: Consultancy, Research Funding; Picnic Health: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Research Funding; MediCom Worldwide, Inc.: Honoraria; Genentech, Inc.: Consultancy, Research Funding; Medscape: Honoraria; Physicians Educational Resource: Honoraria; American Society of Hematology: Honoraria; Astex Pharmaceuticals: Research Funding; Blueprint Medicines, Inc: Research Funding; Partner Therapeutics: Consultancy. Stein:Janssen: Consultancy; Daiichi: Consultancy; Agios: Consultancy; Ono Pharma: Consultancy; Jazz: Consultancy; Syros: Consultancy; Astellas: Consultancy; PinotBio: Consultancy; Blueprint: Consultancy; Novartis: Consultancy; Menarini: Consultancy; Genesis: Consultancy; Calithera: Consultancy; Genentech: Consultancy; Gilead: Consultancy; Syndax: Consultancy; OnCusp: Consultancy; Neoleukin: Consultancy; Abbvie: Consultancy; CTI Biopharma: Consultancy; Foghorn: Consultancy; Servier: Consultancy; Eisai: Research Funding; Bristol Myers Squib: Consultancy, Research Funding; Aptose: Consultancy. Brunner:GSK: Research Funding; Celgene/BMS: Consultancy, Research Funding; AstraZeneca: Research Funding; Agios: Consultancy, Research Funding; Acceleron: Consultancy; Janssen: Research Funding; Keros Therapeutics: Consultancy; Novartis: Consultancy, Research Funding; Taiho: Consultancy; Gilead: Consultancy; Takeda: Consultancy. Zeidner:Sellas: Consultancy; Novartis: Consultancy; Stemline: Research Funding; Shattuck Labs: Honoraria, Research Funding; Immunogen: Honoraria; Gilead: Consultancy, Honoraria, Research Funding; Foghorn: Consultancy; Daiichi Sankyo: Honoraria; Astex: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Arog: Research Funding; Servier: Consultancy, Honoraria; Jazz: Research Funding; Merck: Research Funding; Takeda: Research Funding; Sumitomo Dainippon Pharma: Research Funding. Savona:Geron Corporation: Membership on an entity's Board of Directors or advisory committees; Forma Therapeutics Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma Corp.: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; AbbVie Inc.: Membership on an entity's Board of Directors or advisory committees; Karyopharm Therapeutics Inc.: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Ryvu Therapeutics: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology, Inc.: Membership on an entity's Board of Directors or advisory committees; Taiho: Membership on an entity's Board of Directors or advisory committees; Takeda Pharmaceutical Company: Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Boehringer Ingelheim: Patents & Royalties; ALX Oncology: Research Funding; Astex Pharmaceuticals: Research Funding; Incyte Corporation: Research Funding. Roboz:Agios: Consultancy; Amgen: Consultancy; AbbVie: Consultancy; Actinium: Consultancy; BMS: Consultancy; Janssen: Consultancy, Research Funding; Mesoblast: Consultancy; MEI: Consultancy; Jasper: Consultancy; Jazz: Consultancy; Astellas: Consultancy; AZ: Consultancy; Pfizer: Consultancy; Novartis: Consultancy; GSK: Consultancy; Blueprint: Consultancy; Bluebird bio: Consultancy; Syndax: Consultancy; Takeda: Consultancy. Wang:Gilead: Consultancy; BMS: Consultancy; GlaxoSmithKline: Consultancy; Jazz: Consultancy; Kite: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; PharmaEssentia: Consultancy; Takeda: Consultancy; Dava oncology: Speakers Bureau; Kura Oncology: Speakers Bureau; Astellas: Consultancy, Speakers Bureau; Abbvie: Consultancy. Shallis:Bristol Myers Squibb: Consultancy; Rigel: Consultancy; Servier: Consultancy; Curio Science: Consultancy; Gilead Sciences: Consultancy. Xie:Moffitt Cancer Center: Current Employment; Novartis: Speakers Bureau. Padron:Kura: Research Funding; CTI: Membership on an entity's Board of Directors or advisory committees; Pharmaessentia: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Incyte: Research Funding; Gillead: Membership on an entity's Board of Directors or advisory committees. Maciejewski:Novartis: Honoraria, Speakers Bureau; Regeneron: Consultancy, Honoraria; Alexion: Membership on an entity's Board of Directors or advisory committees; Omeros: Consultancy. Sallman:Aprea, Jazz: Research Funding; AbbVie, Affimed Gmbh, Gilead, Incyte, Intellisphere, LLC, Molecular Partners AG, PGEN Therapeutics, Inc., Takeda, Zentalis; Advisory board for AvenCell, BlueBird Bio, BMS, Intellia, Jasper Therapeutics, Kite, Magenta Therapeutics, NKARTA, Novartis, Orbita: Consultancy. Della Porta:Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Komrokji:Rigel, Taiho, DSI: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Geron: Consultancy; AbbVie, CTI biopharma, Jazz, Pharma Essentia, Servio: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Zeidan:Regeneron: Consultancy, Honoraria; Astex: Research Funding; Otsuka: Consultancy, Honoraria; Kura: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; BioCryst: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Shattuck Labs: Research Funding; Zentalis: Consultancy, Honoraria; Orum: Consultancy, Honoraria; Syros: Consultancy, Honoraria; Notable: Consultancy, Honoraria; Taiho: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Geron: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; BeyondSpring: Consultancy, Honoraria; Boehringer-Ingelheim: Consultancy, Honoraria; Tyme: Consultancy, Honoraria; Schrödinger: Consultancy, Honoraria; Foran: Consultancy, Research Funding; Servier: Consultancy, Honoraria; Agios: Consultancy, Honoraria; Ionis: Consultancy, Honoraria; Mendus: Consultancy, Honoraria; Syndax: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Lox Oncology: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; ALX Oncology: Consultancy, Honoraria; Chiesi: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal